Background:
For patients with relapsed and refractory (R/R) classical Hodgkin lymphoma (HL), the standard of care remains salvage therapy followed by autologous stem cell transplant (ASCT) for responding patients. Historical data suggest 40% of patients may relapse after ASCT, and these patients have generally a poor median overall survival (OS) of less than 2 years (Moskowitz et al., Br J Haematol. 2009;146(2):158-62). However, since these reports, the treatment landscape of R/R HL has dramatically improved, most notably with the introduction of two novel therapies, brentuximab vedotin (BV) and anti-PD1 agents. To refine our understanding of clinical outcomes in this context, we characterized patients who relapsed after ASCT in the modern era.
Methods:
We used a multicenter retrospective patient database at 5 transplant centers in the United States to identify patients with HL aged ≥ 12 years at diagnosis who had received ASCT on or after 1/1/2010. The primary objective was to determine the median OS for patients who relapsed after ASCT (post-ASCT-relapse OS), defining survival as the time from relapse after ASCT to subsequent death from any cause. Univariable Cox regression was performed to evaluate the impact of various factors on post-ASCT OS. Outcomes by era were evaluated using three different eras based on approval dates of novel agents: (1) pre-BV approval (pre-7/31/2011), (2) post-BV approval (8/1/2011-4/30/2016), (3) post-anti-PD1 approval (5/1/16-present).
Results:
A total of 215 patients were identified. The median age at ASCT was 34. Before ASCT, 128 patients (62%) had no exposure to novel agents, 76 patients (35%) had received BV and no anti-PD-1 therapy, and 11 patients (5.1%) had received anti-PD-1 therapy with or without BV. Conditioning was mostly with BEAM (59%) or CBV (32%). The median time to relapse after ASCT was 6 months (range: 1-109 months). Nearly half all relapses (47%) occurred within 6 months of ASCT. Only 14% of relapses occurred after 1 year. Among 126 patients with available treatment information, the median number of post-ASCT lines of therapy was 2 (IQR: 1, 4). Therapy for post-ASCT relapse included BV and no anti-PD-1 therapy in 41 patients (29%), anti-PD-1 therapy with or without BV for 70 patients (50%), and no novel agents for only 15 patients (11%).
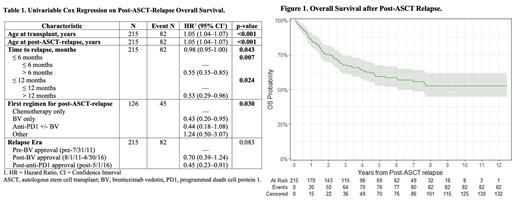
The median followup time from the date of ASCT among survivors was 5.0 (IQR: 2.8, 7.8) years. The median post-ASCT-relapse OS was not reached ( Figure 1). The 2- and 5-year post-ASCT-relapse OS were 75% (95% CI: 70%-81%) and 59% (95% CI: 53%-67%), respectively. On univariable Cox regression for post-ASCT-relapse OS ( Table 1), time to relapse greater than 6 (HR 0.55, 95% CI 0.35-0.85) or 12 months (HR 0.53, 95% CI 0.29-0.96) were significantly associated with improved post-ASCT-relapse OS. Increasing age at relapse was associated with worse OS (HR 1.05, 95% CI 1.04-1.07). Receipt of a novel agent (compared to chemotherapy alone) as the first salvage regimen for post-ASCT relapse was associated with improved post-ASCT OS (BV: HR 0.43, 95% CI 0.20-0.96; anti-PD-1: HR 0.44, 95% CI 0.18-1.08). Outcomes improved over time as regulatory approvals for novel agents occurred (post-BV-approval: HR 0.70, 95% CI 0.39-1.24; post-anti-PD-1 approval: HR 0.45, 95% CI 0.23-0.91).
Conclusion:
We show prolonged overall survival post-ASCT relapse compared to historical data likely due to the introduction of BV and anti-PD1 agents. This report represents one of the largest datasets of post-ASCT relapse in cHL, and this data should serve as an updated benchmark for future evaluations.
Disclosures
Desai:Seagen: Honoraria. Herrera:Genmab: Consultancy; Caribou Biosciences: Consultancy; Tubulis GmbH: Consultancy; BMS: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding; AstraZeneca/MedImmune: Consultancy; Pfizer: Consultancy; Adicet Bio: Consultancy; Karyopharm Therapeutics: Consultancy; Allogene Therapeutics: Consultancy; Regeneron: Consultancy; AbbVie: Consultancy; Kite, a Gilead Company: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Takeda: Consultancy; Genentech/Roche: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Gilead Sciences: Research Funding; AstraZeneca: Research Funding. Mei:Incyte: Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; Seagen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; EUSA: Membership on an entity's Board of Directors or advisory committees; Beigene: Research Funding; Morphosys: Research Funding; BMS: Research Funding. Merryman:Genentech/Roche: Research Funding; Epizyme: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Intellia: Membership on an entity's Board of Directors or advisory committees; Alphasights: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding. Moskowitz:Incyte: Research Funding; Beigene: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Honoraria, Research Funding; Merck: Honoraria, Research Funding; ADC Therapeutics: Research Funding.